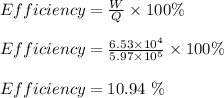Answer:
(a) The magnitude of the change in internal energy is 6.623 x 10⁵ J
(b) the efficiency of the athlete is 10.94 %
Step-by-step explanation:
Given;
work done by the athlete (system), W = 6.53 x 10⁴ J
the heat given off by the athlete (system), Q = 5.97 x 10⁵ J
The simple diagram below will be used to illustrate the direction of the energy flow assuming a heat engine.
Q← ⊕ →W
The work, W, points away from the system since the system does the work
The heat, Q, points away from the system since heat is given off
Apply first law of thermodynamic;
ΔU = Q + W
where;
q is the heat flowing into or out of the system
(+q if the heat is flowing into the system
(-q if the heat is leaving the system
w is the work done by or on the system
(+w if the work is done on the system by the surrounding
(-w if the work is done by the system to the surrounding
Thus, from the above explanation, the change in internal energy of the system is calculated as;
ΔU = -Q - W
ΔU = - 5.97 x 10⁵ J - 6.53 x 10⁴ J
ΔU = -6.623 x 10⁵ J
The magnitude of the change in internal energy = 6.623 x 10⁵ J
(b) the efficiency of the athlete;