Answer: The statement, chemicals that you begin with found on the left side of the equation, describes reactant and where they are in a chemical equation.
Step-by-step explanation:
An equation that includes symbolic formulas and represents chemical reaction between the substances initially present forming new substances is called a chemical equation.
For example,
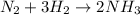
Here, substances
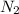 and
and
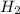 are the reactants. Reactants in a chemical equation are always present on the left side as these are the chemicals we begin with.
are the reactants. Reactants in a chemical equation are always present on the left side as these are the chemicals we begin with.
Also here,
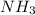 is the product. Product(s) in a chemical equation are always present on the right side as these are the chemicals we end with.
is the product. Product(s) in a chemical equation are always present on the right side as these are the chemicals we end with.
Thus, we can conclude that the statement, chemicals that you begin with found on the left side of the equation, describes reactant and where they are in a chemical equation.