Answer:
The mass of the juice responsible for melting the ice is 949.043 grams.
Step-by-step explanation:
By the First Law of Thermodynamics, we understand that juice releases heat to the ice, which turns into water under the assumption that interactions between the ice-juice system and surroundings are negligible and energy processes are done in steady-state. Since juice is done with water, its specific heat will be taken as of the water. The process is described by the following formula:
![m_(i) \cdot [c_(i)\cdot (T_(1)-T_(2)) - L_(f) + c_(w)\cdot (T_(2)-T_(3))] + m_(w) \cdot c_(w)\cdot (T_(4)-T_(3)) = 0](https://img.qammunity.org/2022/formulas/physics/college/tfr87wuat4vwd38vu4ahiuwwspi8qv1t1x.png) (1)
(1)
Where:
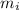 - Mass of ice, in grams.
- Mass of ice, in grams.
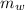 - Mass of the juice, in grams.
- Mass of the juice, in grams.
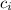 - Specific heat of ice, in joules per gram-degree Celsius.
- Specific heat of ice, in joules per gram-degree Celsius.
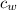 - Specific heat of water, in joules per gram-degree Celsius.
- Specific heat of water, in joules per gram-degree Celsius.
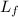 - Latent heat of fusion, in joules per gram.
- Latent heat of fusion, in joules per gram.
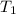 - Initial temperature of ice, in degrees Celsius.
- Initial temperature of ice, in degrees Celsius.
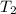 - Melting point of water, in degrees Celsius.
- Melting point of water, in degrees Celsius.
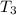 - Final temperature of the ice-juice system, in degrees Celsius.
- Final temperature of the ice-juice system, in degrees Celsius.
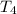 - Initial temperature of the juice, in degrees Celsius.
- Initial temperature of the juice, in degrees Celsius.
If we know that
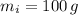 ,
,
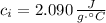 ,
,
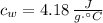 ,
,
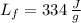 ,
,
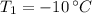 ,
,
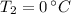 ,
,
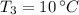 and
and
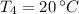 , then the mass of the juice is:
, then the mass of the juice is:
![m_(w) = (m_(i)\cdot [c_(i)\cdot (T_(1)-T_(2)) - L_(f) + c_(w)\cdot (T_(2)-T_(3))])/(c_(w) \cdot (T_(3)-T_(4)))](https://img.qammunity.org/2022/formulas/physics/college/sdibdr7rhz8658hofh7vayi87vch3hwyxi.png)
![m_(w) = ((100\,g)\cdot \left[\left(2.090\,(J)/(g\cdot ^(\circ)C) \right)\cdot (-10\,^(\circ)C) - 334\,(J)/(g) +\left(4.18\,(J)/(g\cdot ^(\circ)C) \right)\cdot (-10\,^(\circ)C) \right])/(\left(4.180\,(J)/(g\cdot ^(\circ)C) \right)\cdot (-10\,^(\circ)C))](https://img.qammunity.org/2022/formulas/physics/college/r8qbqogxonfy4chq0rmwuuh7rjzedx9lhl.png)
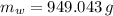
The mass of the juice responsible for melting the ice is 949.043 grams.