Answer:
If the pressure is adjusted to 6.4 atm, the volume will be 17.47 atm.
Step-by-step explanation:
Boyle's law says that the volume occupied by a given gaseous mass at constant temperature is inversely proportional to pressure. That is, if the pressure increases, the volume decreases while if the pressure decreases, the volume increases.
Mathematically, Boyle's law indicates that the product of pressure and volume always has the same value:
P*V= k
Analyzing an initial state 1 and a final state 2, it is satisfied:
P1*V1= P2*V2
In this case:
- P1= 17.2 atm
- V1= 6.5 L
- P2= 6.4 atm
- V2= ?
Replacing:
17.2 atm* 6.5 L= 6.4 atm* V2
Solving:
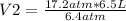
V2=17.47 L
If the pressure is adjusted to 6.4 atm, the volume will be 17.47 atm.