Answer:
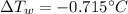
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible to realize that the heat relationship between iron and water is given by:
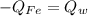
Which in terms of mass, specific heat and temperature is:
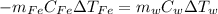
Thus, by solving for the change in the temperature of water we will obtain:
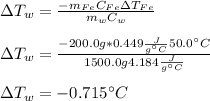
Which is negative because iron increases its temperature and therefore water decreases it.
Regards!