Answer:
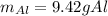
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction:
2 Al + 3 Cl2 --> 2 AlCl3
Whereas there is a 2:3 mole ratio of aluminum to chlorine; it will be possible for us to calculate the required grams of aluminum by using the equality 22.4 L = 1 mol, the aforementioned mole ratio and the atomic mass of aluminum (27.0 g/mol) to obtain:
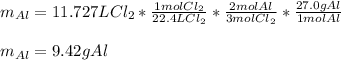
Regards!