Answer:
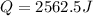
Step-by-step explanation:
Hello there!
In this case, for this general calorimetry problem, it turns out possible for us to calculate the required heat by using the general heat equation in terms of the mass, specific heat and temperature change:
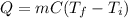
Thus, by plugging in the mass, the initial and final temperatures and the specific heat of ice, 2.05 in SI units, we obtain:
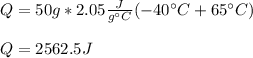
Regards!