Answer: There will be 41382 J energy must be absorbed by 45.0g of water to increase its temperature from
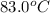 to
to
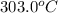 .
.
Step-by-step explanation:
Given: Mass = 45.0 g
Initial temperature =
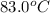
Final temperature =
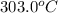
Formula used to calculate heat energy is as follows.
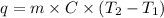
where,
q = heat energy
m = mass of substance
C = specific heat =
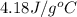 (here, for water)
(here, for water)
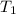 = initial temperature
= initial temperature
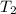 = final temperature
= final temperature
Substitute the values into above formula as follows.
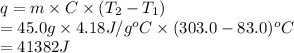
Thus, we can conclude that there will be 41382 J energy must be absorbed by 45.0g of water to increase its temperature from
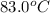 to
to
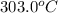 .
.