Answer: An Arrhenius acid is a type of substance yields hydrogen ions,
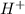 , in an aqueous solution.
, in an aqueous solution.
Step-by-step explanation:
Substances that give dissociate to give hydrogen ions or protons when dissolved in an aqueous solution are called Arrhenius acid.
For example, HCl is a strong acid and upon dissociation in water it gives the following ions.
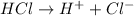
Thus, we can conclude that an Arrhenius acid is a type of substance yields hydrogen ions,
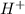 , in an aqueous solution.
, in an aqueous solution.