Answer:
The temperature is 81.3 K.
Step-by-step explanation:
An ideal gas is a theoretical gas that is considered to be composed of point particles that move randomly and do not interact with each other. Gases in general are ideal when they are at high temperatures and low pressures.
The pressure, P, the temperature, T, and the volume, V, of an ideal gas, are related by a simple formula called the ideal gas law:
P*V = n*R*T
where P is the gas pressure, V is the volume that occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas. The universal constant of ideal gases R has the same value for all gaseous substances.
In this case:
- P= 2.5 atm
- V= 8 L
- n=3 moles
- R= 0.082
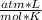
- T= ?
Replacing:
2.5 atm* 8 L= 3 moles* 0.082
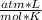 *T
*T
Solving:
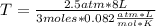
T= 81.3 K
The temperature is 81.3 K.