Answer: The
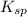 value for calcium hydroxide at this temperature is
value for calcium hydroxide at this temperature is
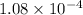 .
.
Step-by-step explanation:
Given: Mass of
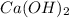 = 0.225 g
= 0.225 g
Volume = 0.100 L
As moles is the mass of substance divided by its molar mass.
So, moles of
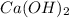 (molar mass = 74 g/mol) is calculated as follows.
(molar mass = 74 g/mol) is calculated as follows.
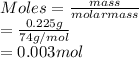
Molarity is the number of moles of substance present in a liter of solution.
Hence, molarity of given solution will be as follows.
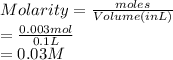
The equation for dissociation of
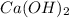 is as follows.
is as follows.
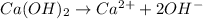
This means that
![[Ca^(2+)] = 0.03](https://img.qammunity.org/2022/formulas/chemistry/college/myp4vdbk0davsjkxo7v1pz30qln4k54pr7.png) and
and
![[OH^(-)] = 2 * 0.03 = 0.06](https://img.qammunity.org/2022/formulas/chemistry/college/n3198udowee9hk3290xuhal8uz0c7u7cxa.png) . Hence,
. Hence,
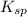 value for this reaction is calculated as follows.
value for this reaction is calculated as follows.
![K_(sp) = [Ca^(2+)][OH^(-)]^(2)\\= (0.03) * (0.06)^(2)\\= 1.08 * 10^(-4)](https://img.qammunity.org/2022/formulas/chemistry/college/10me8mr49i4xf6kqjmbevl4khcbbo8h21g.png)
Thus, we can conclude that the
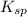 value for calcium hydroxide at this temperature is
value for calcium hydroxide at this temperature is
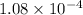 .
.