1.
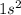 represents helium (He) with atomic number 2.
represents helium (He) with atomic number 2.
2.
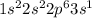 represents sodium (Na) with atomic number 11.
represents sodium (Na) with atomic number 11.
3.
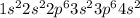 represents calcium (Ca) with atomic number 20.
represents calcium (Ca) with atomic number 20.
4.
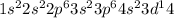 represents scandium (Sc) with atomic number 21.
represents scandium (Sc) with atomic number 21.
5.
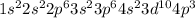 represents phosphorus (P) with atomic number 15.
represents phosphorus (P) with atomic number 15.
1.
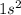 represents helium (He). This electron configuration indicates that helium has two electrons in its first and only energy level. Helium is in period 1, group 18 (noble gases), with an atomic number of 2.
represents helium (He). This electron configuration indicates that helium has two electrons in its first and only energy level. Helium is in period 1, group 18 (noble gases), with an atomic number of 2.
2.
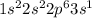 represents sodium (Na). Sodium has electrons distributed in the first three energy levels. It is in period 3, group 1 (alkali metals), with an atomic number of 11.
represents sodium (Na). Sodium has electrons distributed in the first three energy levels. It is in period 3, group 1 (alkali metals), with an atomic number of 11.
3.
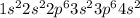 represents calcium (Ca). Calcium has electrons distributed in the first four energy levels. It is in period 4, group 2 (alkaline earth metals), with an atomic number of 20.
represents calcium (Ca). Calcium has electrons distributed in the first four energy levels. It is in period 4, group 2 (alkaline earth metals), with an atomic number of 20.
4.
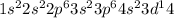 represents scandium (Sc). Scandium has electrons distributed in the first five energy levels. It is in period 4, group 3 (transition metals), with an atomic number of 21.
represents scandium (Sc). Scandium has electrons distributed in the first five energy levels. It is in period 4, group 3 (transition metals), with an atomic number of 21.
5.
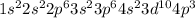 represents phosphorus (P). Phosphorus has electrons distributed in the first five energy levels. It is in period 3, group 15 (pnictogens), with an atomic number of 15.
represents phosphorus (P). Phosphorus has electrons distributed in the first five energy levels. It is in period 3, group 15 (pnictogens), with an atomic number of 15.