Answer:
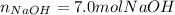
Step-by-step explanation:
Hello there!
In this case, according to the given information about this problem, we first consider the given chemical reaction:
2Na + 2H2O --> 2NaOH + H2
Whereas there is 2:2 mole ratio of NaOH to Na; thus, we calculate the required theoretical yield of NaOH in moles as shown below:
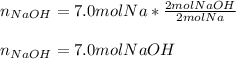
Regards!