Answer:
pH = 0.903.
Step-by-step explanation:
Hello there!
In this case, according to the given information about the problem, we can notice there is a reaction between nitric acid and ammonia, whereas just the H ions from the former are relevant:
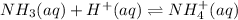
Thus, we first calculate the consumed moles of ammonia, it order to evaluate the moles after the reaction:
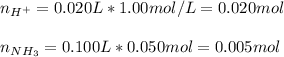
It means there is a leftover of moles of hydrogen ions of 0.015mol; then, we calculate the molarity of the resulting solution as follows:
![[H^+]=(0.015mol)/(0.120L)=0.125M](https://img.qammunity.org/2022/formulas/chemistry/high-school/zu67cepw3d76fwvgvwcfc7x1bm52lnyluc.png)
Finally, we use the formula for the calculation of the pH to obtain:
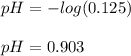
Which means this solution is highly acidic.
Regards!