Answer:
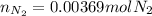
Step-by-step explanation:
Hello there!
In this case, by firstly setting up the described chemical reaction, we are able to write:
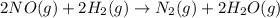
Thus, given the pressure (convert to atm), volume and temperature (convert to K) we can calculate the moles of NO:
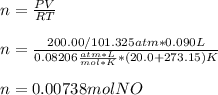
Next, we use the 2:1 mole ratio of NO to N2 to obtain the moles of nitrogen gas that will be produced:
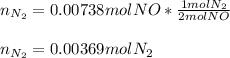
Regards!