Answer:
Final temperature, T2 = 381.28°C
Step-by-step explanation:
Given the following data;
Mass = 6.23 g
Initial temperature = 30.4°C
Heat capacity = 282 J
Specific heat capacity = 0.129 J/g°C
To find the final temperature;
Heat capacity is given by the formula;
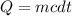
Where;
Q represents the heat capacity or quantity of heat.
m represents the mass of an object.
c represents the specific heat capacity of water.
dt represents the change in temperature.
Making dt the subject of formula, we have;
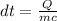
Substituting into the equation, we have;
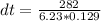
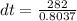
dt = 350.88°C
Now, the final temperature T2 is;
But, dt = T2 - T1
T2 = dt + T1
T2 = 350.88 + 30.4
Final temperature, T2 = 381.28°C