Answer: There are 576.46 number of grams present in 16.95 mol hydrogen peroxide
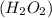 .
.
Step-by-step explanation:
Number of moles is defined as the mass of substance divided by its molar mass.
The molar mass of
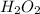 is 34.01 g/mol. Hence, mass of hydrogen peroxide present in 16.95 moles is calculated as follows.
is 34.01 g/mol. Hence, mass of hydrogen peroxide present in 16.95 moles is calculated as follows.
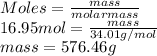
Thus, we can conclude that there are 576.46 number of grams present in 16.95 mol hydrogen peroxide
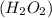 .
.