Answer: If a substance has a boiling point of
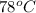 then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
Step-by-step explanation:
The temperature at which vapor pressure of a liquid substance becomes equal to the atmospheric pressure is called boiling point of substance.
At the boiling point, liquid phase and vapor phase remains in equilibrium.
This means that as liquid phase changes into vapor phase and also vapor phase changes into liquid phase at the boiling point.
Thus, we can conclude that if a substance has a boiling point of
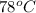 then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.