Answer:
More energy is transferred in situation A
Step-by-step explanation:
Each of the situations are analyzed as follows;
Situation A
The temperature of the cup of hot chocolate = 40 °C
The temperature of the interior of the freezer in which the chocolate is placed = -20 °C
We note that at 0°C, the water in the chocolate freezes
The energy transferred by the chocolate to the freezer before freezing is given approximately as follows;
E₁ = m×c₁×ΔT₁
Where;
m = The mass of the chocolate
c₁ = The specific heat capacity of water = 4.184 kJ/(kg·K)
ΔT₁ = The change in temperature from 40 °C to 0°C
Therefore, we have;
E₁ = m×4.184×(40 - 0) = 167.360·m kJ
The heat the coffee gives to turn to ice is given as follows;
E₂ = m·
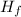
Where;
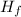 = The latent heat of fusion = 334 kJ/kg
= The latent heat of fusion = 334 kJ/kg
∴ E₂ = m × 334 kJ/kg = 334·m kJ
The heat required to cool the frozen ice to -20 °C is given as follows;
E₃ = m·c₂·ΔT₂
Where;
c₂ = The specific heat capacity of ice = 2.108 kJ/(kg·K)
Therefore, we have;
E₃ = m × 2.108 ×(0 - (-20)) = 42.16
E₃ = 42.16·m kJ/(kg·K)
The total heat transferred = (167.360 + 334 + 42.16)·m kJ/(kg·K) = 543.52·m kJ/(kg·K)
Situation B
The temperature of the cup of hot chocolate = 90 °C
The temperature of the room in which the chocolate is placed = 25 °C
The heat transferred by the hot cup of coffee, E, is given as follows;
E = m×4.184×(90 - 25) = 271.96
∴ E = 271.96 kJ/(kg·K)
Therefore, the total heat transferred in situation A is approximately twice the heat transferred in situation B and is therefore more than the heat transferred in situation B
Energy transferred in situation A = 543.52 kJ/(kg·K)
Energy transferred in situation B = 271.96 kJ/(kg·K)
Energy transferred in situation A ≈ 2 × Energy transferred in situation B
∴ Energy transferred in situation A > Energy transferred in situation B.