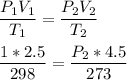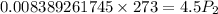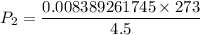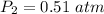Answer:
P_2 =0.51 atm
Step-by-step explanation:
Given that:
Volume (V1) = 2.50 L
Temperature (T1) = 298 K
Volume (V2) = 4.50 L
at standard temperature and pressure;
Pressure (P1) = 1 atm
Temperature (T2) = 273 K
Pressure P2 = ??
Using combined gas law: