Answer:
V₂ = 4.00 L
Step-by-step explanation:
Given that:
Volume (v1) = 6.00 L
Temperature (T1) = 300 K
Pressure (P1) = 1.00 atm
VOlume (V2) = unknown???
Temperature (T2) = 600 K
Pressure (P2) = 3.00 atm
Using combined gas law equation:
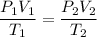
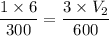
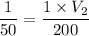
200 = 50V₂
V₂ = 200/50
V₂ = 4.00 L