Answer:

Step-by-step explanation:
To convert from moles to grams, the molar mass is used (mass of 1 mole). The values are the same as the atomic masses on the Periodic Table, but the units are grams per mole (g/mol) instead of atomic mass units.
1. Molar Mass
We are given the compound sodium hydroxide (NaOH) and we need to look up the molar masses of the individual elements.
- Na: 22.9897693 g/mol
- O: 15.999 g/mol
- H: 1.008 g/mol
The formula for the compound has no subscripts, so there is 1 mole of each element in 1 mole of the substance. We can simply add the molar masses.
- NaOH: 22.9897693 + 15.999 + 1.008 = 39.9967693 g/mol
This means there are 39.9967693 grams of sodium hydroxide in 1 mole.
2. Convert Grams to Moles
Use the molar mass we found as a ratio.
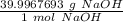
Since we are converting 17.6 grams of NaOH to moles, we multiply by this value.
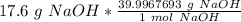
Flip the ratio so the units of grams of NaOH cancel.
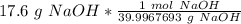
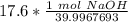
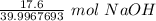
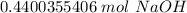
3. Round
The original measurement of grams has 3 significant figures, so our answer must have the same. For the number we calculated, that is the thousandth place.
The 0 in the ten thousandths place (in bold above) tells us to leave the 0 in the thousandth place.
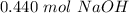
17.6 grams of sodium hydroxide are equal to 0.440 moles of sodium hydroxide.