Answer:

Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to calculate the freezing point of an aqueous solution of potassium sulfide by using the following equation:

In such a way, we firstly calculate the molality of this solution according to:
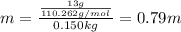
Finally, since the Van't Hoff's factor for K2S is 3, the freezing point of the solution turns out to be:
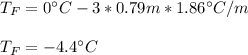
Regards!