Answer:
M = 1.4 M
Step-by-step explanation:
Hello there!
In this case, according to the given information, it will be possible for us to calculate the required molarity according to its definition:
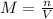
Whereas the n is mole and has a value of 0.7 mol, V the volume in liters, which is 0.5 L; therefore, the result is:
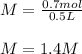
Regards!