Answer:
(a) pH=4.0
(b) pH=8.3
Step-by-step explanation:
Hello there!
In this case, since the pH is understood as the potential of the hydrogen in an aqueous solution, and we can calculate it is as follows:
![pH=-log([H^+])](https://img.qammunity.org/2022/formulas/chemistry/high-school/udp5mrf49k7gzi60hxhr2bxzaatpy3tthi.png)
We simply need to plug in the concentrations on each question as shown below:
(a)
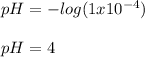
(b)
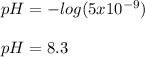
Regards!