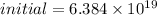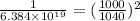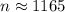Answer:
The appropriate solution is:
(a) n ≈ 900
(b) n ≈ 1165
Step-by-step explanation:
According to the question,
(a)
The final number of molecules throughout water will be:
=
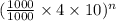
where, n = number of extractions
Now,
The initial number of molecules will be:
=
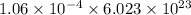
=
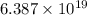
Final number of molecule,
⇒
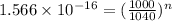
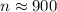
(b)
Final molecules of X = left (0.01%)
hence,
⇒