Answer:
Formula: Na2S2O3
we get solubility.
Divide the mass of the compound by the mass of the solvent and then multiply by 100 g to calculate the solubility in g/100g .
Solution given:
mass of sodium thiosulphate [m1]=25.5g
mass of water [m2]=40g
at temperature [t]=25°C
we have
solubility in g/dm^3 :
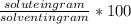
- =
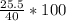
- =63.75g /litre=63.75g/dm³
solubility in g/dm^3 :63.75g/dm³
now
solubility of the solute in mol/dm^3=:63.75g/dm³/178=0.4 mol/dm³