Answer:
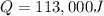
Step-by-step explanation:
Hello there!
In this case, since the vaporization process is carried out in order to turn a liquid into a gas due to the addition of heat, we can use the following heat equation involving the heat of vaporization of water or any other substance:
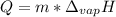
Thus, since this heat of vaporization for water is 2259.36 J/g, we plug in this amount to obtain the total energy for this process.
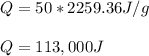
Which is positive due to the necessity of heat.
Regards!