Answer:
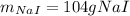
Step-by-step explanation:
Hello there!
In this case, given the balanced chemical reaction, it is possible to evidence that the mole ratio of sodium iodide to iodine is 2:1 and the molar masses are 149.89 and 253.81 g/mol respectively; in such a way, we write the following stoichiometric setup in order to obtain the required grams of sodium iodide:
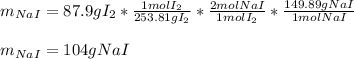
Regards!