Answer:
1 - Salts required that can be compared directly, using Ksp values, to estimate solubilities for copper (II) sulfide are -
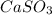 (Option b) ,
(Option b) ,
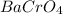 (Option c) and
(Option c) and
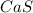 (Option d)
(Option d)
2 - Salts that can be compared directly, using Ksp values, to estimate solubilities for zinc hydroxide are -
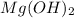 (Option a)
(Option a)
Step-by-step explanation:
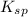 values can be used to compare the solubility of salts that produce ions in a 1:1 ratio.
values can be used to compare the solubility of salts that produce ions in a 1:1 ratio.
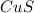 {copper (II)sulfide} dissociates with ions in ratio 1:1 .
{copper (II)sulfide} dissociates with ions in ratio 1:1 .
Because the salts
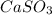 ,
,
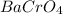 and
and
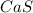 have a 1:1 ion ratio, the solubilities of options b, c, and d can be compared to salt 1.
have a 1:1 ion ratio, the solubilities of options b, c, and d can be compared to salt 1.
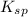 Values can be used to compare the solubility of salts that produce ions in a 1:2 or 2:1 ratio.
Values can be used to compare the solubility of salts that produce ions in a 1:2 or 2:1 ratio.
2.
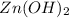 dissociates into ions in ratio 1:2 .
dissociates into ions in ratio 1:2 .
Because the ions in the salt
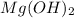 are in a 1:2 ratio, the solubility of salt 2 can be compared to that of salt 'a'.
are in a 1:2 ratio, the solubility of salt 2 can be compared to that of salt 'a'.
Hence , Salt 1 is matched with options b , c , d.
Salt 2 is matched with option a.