Answer:
The pressure before expansion is 717.1 kPa.
Step-by-step explanation:
We need to use the Ideal Gas equation to find the initial pressure:
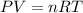
Where:
P: is the pressure
V: is the volume
n: is the number of moles
R: is the gas constant
T: is the temperature
Since the number of moles does not change after the expansion, we need the following number of moles:
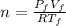 (1)
(1)
Where "f" is for final
Before the expansion, the pressure is:
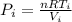 (2)
(2)
By entering equation (1) into (2) we have:
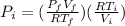
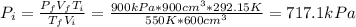
Therefore, the pressure before expansion is 717.1 kPa.
I hope it helps you!