Answer:
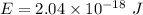
Step-by-step explanation:
We need to find the energy for an electron to jump from n = 1 to n = 4.
The energy in transition from 1 state to another is given by :
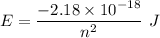
The difference in energy for n = 1 to n = 4 is:
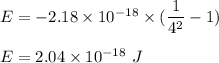
So, the required energy is equal to
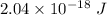 .
.