Answer:
The empirical formula of the compound is
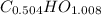 .
.
Step-by-step explanation:
We need to determine the empirical formula in its simplest form, where hydrogen (
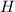 ) is scaled up to a mole, since it has the molar mass, and both carbon (
) is scaled up to a mole, since it has the molar mass, and both carbon (
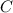 ) and oxygen (
) and oxygen (
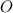 ) are also scaled up in the same magnitude. The empirical formula is of the form:
) are also scaled up in the same magnitude. The empirical formula is of the form:
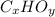
Where
 ,
,
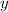 are the number of moles of the carbon and oxygen, respectively.
are the number of moles of the carbon and oxygen, respectively.
The scale factor (
 ), no unit, is calculated by the following formula:
), no unit, is calculated by the following formula:
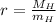 (1)
(1)
Where:
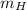 - Mass of hydrogen, in grams.
- Mass of hydrogen, in grams.
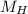 - Molar mass of hydrogen, in grams per mole.
- Molar mass of hydrogen, in grams per mole.
If we know that
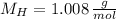 and
and
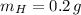 , then the scale factor is:
, then the scale factor is:
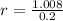
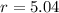
The molar masses of carbon (
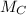 ) and oxygen (
) and oxygen (
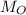 ) are
) are
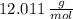 and
and
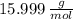 , then, the respective numbers of moles are: (
, then, the respective numbers of moles are: (
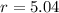 ,
,
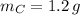 ,
,
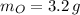 )
)
Carbon
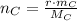 (2)
(2)
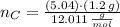
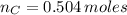
Oxygen
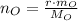 (3)
(3)
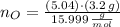
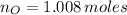
Hence, the empirical formula of the compound is
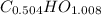 .
.