Given :
- total charge = 9.0 mC = 0.009 C
Each electron has a charge of :
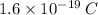
For producing 1 Cuolomb charge we need :
Now, for producing 0.009 C of charge, the number of electrons required is :
_____________________________
So, Number of electrons passing through the cross section in 3.6 seconds is :
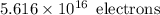
Number of electrons passing through it in 1 Second is :
Now, in 10 seconds the number of electrons passing through it is :
_____________________________