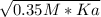Answer:
Ka = 1.14x10⁻⁸
Step-by-step explanation:
First we calculate [H⁺] from the pH:
- [H⁺] =
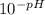
For a monoprotic weak acid, the molar concentration of H⁺ of a solution can be expressed as:
Where C is the molar concentration of the weak acid solution.
- 6.31x10⁻⁵ M =