Answer:
1. 0.82 gram of Ag+
2. 4.79 g of Ag₂O₃S
Step-by-step explanation:
From the given information:
Total amount of Ag₂O₃S = 2.24 grams
Atomic mass of Ag+ =107.86 g/mole
molar mass of Ag₂O₃S = 295.8 g/mole
∴
The mass of the Silver (Ag) in grams is:
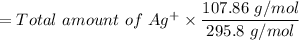
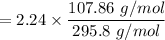
= 0.82 gram of Ag+
2.
Here, the total amount of Ag₂O₃S = unknown
Atomic mass of Ag+ = 107.86 g/mole
molar mass of Ag₂O₃S = 295.8 g/mole
amount of Ag+ = 1.75 g
∴
The mass of Ag₂O₃S =
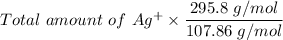
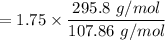
= 4.79 g of Ag₂O₃S