Answer:
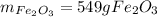
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction for this problem about stoichiometry:
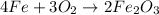
Whereas there is a 3:2 mole ratio of oxygen (molar mass = 32.0 g/mol) to iron (III) oxide (molar mass = 159.69 g/mol) and therefore, the correct stoichiometric setup is:
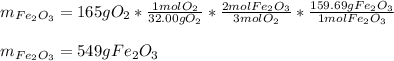
Regards!