________________________________
Using ideal gas law–
We'll use formula,
PV = nRT
Where,
P, Pressure = 1.40 atm
V, Volume = 42.4 L
n, Number of moles = ( To find ) ?
T, temperature = 25°C
R, Ideal gas constant = 0.08206 L . atm/k. Mol
________________________________
Here, PV = nRT
Or, PV / RT = n
Or,
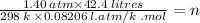
Or, n = 2.43 Moles.
Or, n = 2.43 Moles.
________________________________
I had to take help from my elder brother.
We tried our best, hope it helps you.
By– Debasis ( feat – Apollo)
________________________________