Answer:
True.
Step-by-step explanation:
Hello!
In this case, according to the ideal gas equation:

It turns out possible for us to obtain the Boyle's law, as a directly proportional relationship between pressure and volume, considering that both moles and temperature remain constant; in such a way, we write the following:
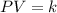
Therefore, for an initial (1) and final state (2) we are able to write:
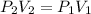
Regards!