Answer:
pH = 12.5
Step-by-step explanation:
The reaction that takes place is
- 2OH⁻(aq) + Pb⁺²(aq) → Pb(OH)₂(s)
The OH⁻ species come from the metal hydroxide of the solution, and Pb(OH)₂ is the precipitate.
Now we convert 3.81 grams of Pb(OH)₂ into moles, using its molar mass:
- 3.81 g ÷ 241.12 g/mol = 0.0158 mol Pb(OH)₂
Now we convert 0.0158 Pb(OH)₂ moles into OH⁻ moles, using the stoichiometric coefficients of the reaction:
- 0.0158 mol Pb(OH)₂ *
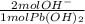 = 0.0316 mol OH⁻
= 0.0316 mol OH⁻
With the given concentration (1 L), we calculate [OH⁻]:
- [OH⁻] = 0.0316 mol / 1 L = 0.0316 M
Then we calculate the pOH of the solution:
And finally we calculate the pH: