Answer:

Step-by-step explanation:
Molarity is a measure of concentration in moles per liter.
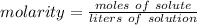
The solution has a molarity of 1.2 M or 1.2 moles per liter. There are 4.0 moles of NaCl, the solute. We don't know the liters of solution, so we can use x.
- molarity= 1.2 mol/L
- moles of solute= 4.0 mol
- liters of solution =x
Substitute the values into the formula.
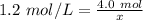
Since we are solving for x, we must isolate the variable. Begin by cross multiply (multiply the 1st numerator and 2nd denominator, then the 1st denominator and 2nd numerator.
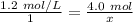
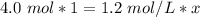
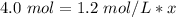
x is being multiplied by 1.2 moles per liter. The inverse of multiplication is division, so divide both sides by 1.2 mol/L
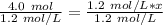
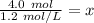
The units of moles (mol) will cancel.
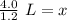
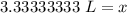
The original measurements both have 2 significant figures, so our answer must have the same. For the number we found, this is the tenths place.
The 3 in the hundredth place tells us to leave the 3 in the tenths place.
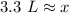
Approximately 3.3 liters of solution are needed to make a 1.2 M solution with 4.0 moles of sodium chloride.