Answer:

Step-by-step explanation:
This problem asks us to find the temperature change necessary to make the volume change. We use Charles's Law which states that temperature is directly proportional to the volume of a gas. The formula is:
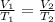
We know the balloon originally had a volume of 6.5 liters and a temperature of 280 Kelvin. Then, the temperature was cooled so the new volume is 3.3 liters. However, the exact new temperature is unknown.
Substitute all known values into the formula.
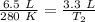
Now, solve the new temperature (T₂). First, cross multiply. Multiply the 1st numerator by the 2nd denominator. Then, multiply the 1st denominator by the 2nd numerator.
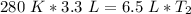
Multiply the left side.
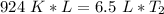
We must isolate the variable. Currently, it is being multiplied by 6.5 liters. The inverse of multiplication is division. Divide both sides by 6.5 L.
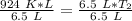
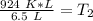
The units of liters (L) cancel.
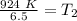
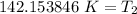
Let's round to the nearest tenth place. The 5 in the hundredth place tells us to round the 1 up to a 2.
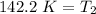
The temperature must be cooled to approximately 142.2 Kelvin.