Answer:
1.765x10²⁵ molecules
Step-by-step explanation:
- C₃H₈ + 5 O₂ → 3 CO₂ + 4H₂O
First we convert 7.328 moles of propane (C₃H₈) into moles of H₂O, using the stoichiometric coefficients of the balanced reaction:
- 7.328 mol C₃H₈ *
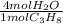 = 29.312 mol H₂O
= 29.312 mol H₂O
Then we convert 29.312 moles of H₂O into molecules, using Avogadro's number:
- 29.312 mol * 6.023x10²³ molecules/mol = 1.765x10²⁵ molecules