Answer:
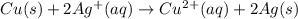
Step-by-step explanation:
Hello there!
In this case, according to the given description of the reaction whereby copper metal reaction a solution of silver nitrate; it turns out possible for us to write out the resulting net ionic equation by firstly write the complete molecular equation:
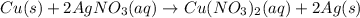
Now, we write the complete ionic equation, by taking into account that just the aqueous species are ionized:
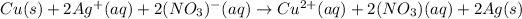
And finally, we cancel out the nitrate ions as the spectator ones in order to obtain:
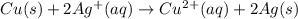
Regards!