Answer: The balanced equation is
 .
.
Step-by-step explanation:
The given reaction equation is as follows.

Number of atoms present on reactant side are as follows.
- Li = 1
- H = 1
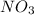 = 1
= 1
Number of atoms present on product side are as follows.
- Li = 1
- H = 2
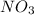 = 1
= 1
To balance this equation, multiply Li by 2 and
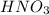 by 2 on reactant side. Also, multiply
by 2 on reactant side. Also, multiply
 by 2 on product side.
by 2 on product side.
Hence, the equation can be rewritten as follows.

Now, number of atoms present on reactant side are as follows.
- Li = 2
- H = 2
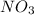 = 2
= 2
Number of atoms present on product side are as follows.
- Li = 2
- H = 2
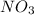 = 2
= 2
As there are same number of atoms on both reactant and product side. Hence, the equation is now balanced.
Thus, we can conclude that the balanced equation is
 .
.