Answer:
n = 12.18 moles
Step-by-step explanation:
Given that,
The volume of a canister, V = 1 L
The temperature of the canister, T = 100 K
Pressure, P = 100 atm
We need to find the number of moles of gas. Let there are n number of moles. We know that,
PV = nRT
Where
R is gas constant, R = 0.0821 L*atm/mol*K
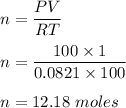
Hence, there are 12.18 moles of gas.