Answer:
number of moles = 0.21120811
Step-by-step explanation:
To find the number of moles, given the mass of the solute, we use the formula:
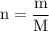
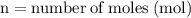
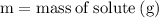
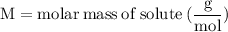
Label the variables with the numbers in the problem:
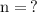
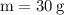
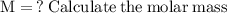
The first thing we have to do is find the molar mass of sodium sulfate, in order for us to use the formula for finding the number of moles:
Formula for finding the molar mass of sodium sulfate:
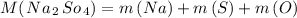
For the variables and what they mean are below for finding the molar mass of sodium sulfate:
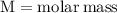
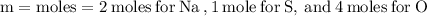
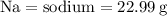
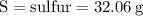
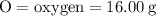
Plug the numbers into the formula, to find the molar mass of sodium sulfate:
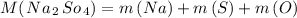
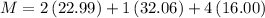
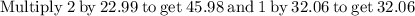
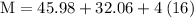
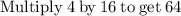
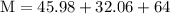
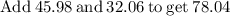
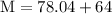
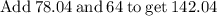
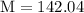
Now that we have found the molar mass, we can calculate the number of moles in the solution of sodium sulfate with the formula:
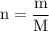
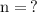
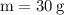
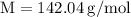
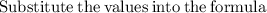
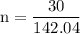
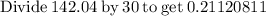
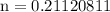
0.21120811 rounded gives you 0.2112
or if you did the problem without decimals
30 grams of sodium sulfate divided by its molecular weight – which we found to be 142 – gives us a value of 0.2113 moles.