Answer:
The answer is:
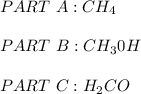
Step-by-step explanation:
Parts A:
The vapor pressure is higher in
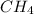 because it is non-polar, while
because it is non-polar, while
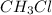 is polar.
is polar.
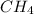 has a lower molar weight as well.
has a lower molar weight as well.
Part B:
Although hydrogen bonding is found in both commodities, the vapor pressure is higher because of the smaller molar mass of
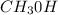 .
.
Part C:
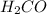 does not show hydrogen due to the increased vapor pressure
does not show hydrogen due to the increased vapor pressure
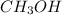 bonding.
bonding.