Answer: The volume of oxygen at STP is needed for complete given combustion is 1121.28 L.
Explanation:
The given reaction equation is as follows.
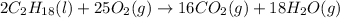
This shows that 2 moles of gasoline requires 25 moles of . Hence, moles of oxygen required to react with 4 moles of gasoline are calculated as follows.
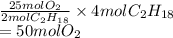
At STP, the pressure is 1 atm and temperature is 273.15 K. Therefore, using ideal gas equation the volume of oxygen is calculated.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
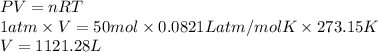
Thus, we can conclude that volume of oxygen at STP is needed for complete given combustion is 1121.28 L.