We are given:
half-life of carbon = 5715 years
Initial mass = 100 grams
Final mass = 12.5 grams
Finding the time taken:
Number of half-lives:
We know that in the relation:
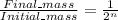 , n is the number of half-lives taken
, n is the number of half-lives taken
replacing the given values:
12.5 / 100 = 1/2ⁿ
1/8 = 1/2ⁿ
2ⁿ = 8
2ⁿ = 2³
n = 3
Hence, it took 3 half-lives to reduce the mass to 12.5 grams
Number of years:
Time taken = 3 half-lives
we know that one half-life is 5715 years, replacing that value:
Time taken = 3*(5715) years
Time taken = 17145 years
Therefore, after 17145 years, a 100 gram sample of carbon will decay and only 12.5 grams will remain